Àêòèâíîñòü ôåðìåíòà Àöåòèëõîëèíýñòåðàçà â ðàçíûõ âîçðàñòíûõ êëåòêàõ ýðèòðîöèòîâ è ñ ïðèìåíåíèåì îêñèìà
Ýäâàðäñ Äæîí,
ïðîôåññîð,
Áåêíàçàðîâà Måðóåðò,
àñïèðàíò.
Ôëèíäåðñêèé Óíèâåðñèòåò, Àäåëàèäà, Þæíàÿ Àâñòðàëèÿ.
Acetylcholinesterase activity in
erythrocytes of different
ages and with oxime treatment
Meruyert Beknazarova,
PhD Candidate,
Supervised by: Associate Professor John Edwards School of the Environment, Flinders University.
Abstract
There are generally three approaches to assess acetylcholinesterase (AChE) inhibition, each with its own disadvantages. These include the lack of AChE pre-exposure data, high AChE intraindividual variation to compare to the reference population data and a long regeneration period for AChE. This study investigated a new method of using oxime to assess regenerated AChE. AChE has been measured in different erythrocyte age cohorts. Plasma cholinesterase (ChE) in whole blood and pure plasma, and AChE in whole blood and mixed, young and old erythrocytes with or without treatment of pralidoxime has been measured in 27 South Australian donors. Oxime has regenerated plasma ChE by almost two fold, however it had an inhibition effect on AChE in each group. Mixed erythrocytes have shown the highest AChE activity as well as the greatest inhibition by oxime. The results of this paper suggest that mixed erythrocytes have higher AChE activities compared to young or old cells. Although oxime did not work as expected in this study, it could still demonstrate AChE is a sensitive target in mixed erythrocytes. Further work is required to examine AChE using different method of erythrocyte fractionation and to further examine the role of oxime in regenerating AChE.
1. Introduction
Pesticides are chemical substances used to prevent, destroy or control pests and are registered for wide use in public health applications, insect control on food crops and residential use in Australia and worldwide (Farahat et al., 2003; Calvert et al., 2004). Pesticides use worldwide has increased substantially over time with pesticides used mostly in agriculture. Thus, according to the pesticide use statistics, approximately 5.05 billion pounds pesticides were used in agriculture in 2011 (Lah, 2011). The latest data on Environment Protection Agency (EPA), US, show the worldwide consumption of pesticides in 2007 came to about 5,6 billion pounds (Grube et al., 2011). Over the last twenty years Australian agriculture and management have expanded largely. The essential attribute to such progress was the treatment of plant and animal pests and diseases. Over the last years Australian use of pesticides has increased, and according to Radcliffe, 2002, Australians use around 10, 000 tonnes of insecticides annually (as at 25 July 2001) (Radcliffe, 2002).
The general population can be exposed to various pesticides through inhalation, ingestion, eye contact or dermal absorption (Griffin et al., 1999), with health consequences ranging from none to mild, or serious (Calvert et al., 2004; Hernandez et al., 2006).
Organophosphate (OP) pesticides are used extensively with many different OPs synthesised and more than two hundred being used (Marrs, 2007). The use of OPs varies from country to country depending on climate, regulations and controls (Muldoon & Hodgson, 1992).
Toxicity of OP compounds is reflected as inhibition of acetylcholinesterase (AChE) and plasma cholinesterase (ChE) (Padilla et al., 1994; Thierman et al., 2007; Elersek & Filipic, 2011). ChE enzymes hydrolyse the neurotransmitter acetylcholine (ACh) in cholinergic synapses (Mishra, 2006; Ren et al., 2014). OPs bind to the active site of AChE by the covalent bonds blocking AChE function. This causes ACh to increase transmitting nerve impulses and causing constant muscle contractions leading eventually to exhaustion and tetany (Gupta, 2006).
Measurements of AChE and plasma ChE have been widely used as biomarkers for potential exposure to OPs, with AChE used as a better predictive indicator of OP clinical risk and plasma ChE as a sensitive index of exposure to OPs (Carlock et al., 1999; Hatijan et al., 2000; Mason, 2000; Dyer et al., 2001; Kamel & Hoppin, 2004; Chowdhary et al., 2014).
The Ellman method is the most used to measure ChEs, where the hydrolysis of acetylthiocholine is measured colorimetrically (Wilson et al., 2002). This has led to the development of several rapid field testing kits (eg Test Mate, Cincinnatti).
OPs are known to cause acute intoxications of occupationally exposed workers (Wesseling et al. 1997; Calvert et al. 2004). Accidental or intentional acute pesticide poisonings occur annually with majority in developing countries (Eddleston et al., 2002; Jaga & Dharmani, 2003), and cause around 200,000 deaths per year globally (Eddleston, 2000; Buckley et al., 2004; Gunnel et al., 2007).
Clinical symptoms of acute OP toxicity are mostly produced during short-term high-dose exposures characterised by acute inhibition of AChE in the brain, neuromuscular junction and peripheral nerves (Hatjian et al., 2000; Hernanez et al., 2006; Chowdhary et al., 2014).
Some studies have also shown that chronic exposures to moderate or low-doses of OPs do not always cause clinical manifestations, while inhibiting plasma ChE and especially AChE (Lakew & Mekonnen, 1998; Hatjian et al., 2000; Ren et al., 2014). Inhibition by 70-90% may not be accompanied by any clinical effects (Hatjian et al., 2000; Dyer et al., 2001). The problem here is that one can be exposed to OPs and have AChE inhibited by up to 70% with no clinical symptoms produced. This leaves little margin of safety for accidental exposure inhibiting the enzyme further and causing clinical effects. Furthermore, long-term exposures to OPs have been associated with developmental neurotoxicity leading to death of a developing organism, structural abnormality, altered growth and functional deficiency (Pope et al., 1991; Costa, 1996; Gupta et al., 2001; Levin et al., 2006; Slotkin et al., 2006), and cancer (Brown et al., 1990; Waddel et al., 2001).
Paradoxically, chronic low-dose or moderate exposures may cause clinical symptoms without inhibition of ChE enzymes (Bushnell & Moser, 2006; cited in Lein et al., 2012, p. 660). Studies on workers exposed to OPs have found symptoms produced without any inhibition of AChE (Maizlish et al., 1987; Parron et al., 1996; Farahat et al., 2003).
Once nicotinic receptors have been overstimulated, oxime application is used to reactivate inhibited ChE (Thierman et. al., 2007). There are two clinically used oximes: pralidoxime (PAM) and obidoxime (LuH-6, Toxogonin), the first being the wider used. Oxime reactivation of AChE is possible only when the inhibition is reversible (no aging); otherwise, AChE has to be resynthesised for recovery (Roberts & Brett, 2014).
It is universally agreed, that erythrocyte senescence is accompanied with the increase in cell density (mass/volume) as cytoplasmic lighter constituents of the cells are preferentially got lost (Sutera et al., 1985). AChE has been measured in reticulocytes and found to be much higher than in mature erythrocytes (Sabine, 1940; Pritchard, 1949; Allison & Burn, 1955) being a function of erythrocyte ageing.
Although the AChE activity and erythrocyte ageing association had been studied decades ago, there are not many studies available, nor have any recommendations on practical application of these observations been done. The lack of one standard AChE measurement method might be one of the reasons for discrepancies in results found.
The aim of the current research is to determine whether younger erythrocytes have the highest AChE activity and recovery rate, and whether more sensitive AChE in younger erythrocytes can be exploited to improve OP exposure assessment methods in field studies. In addition, we may use oxime regeneration to determine baseline AChE activity, which may be useful in improving the assay methodology.
2. Materials and Methods
2.1 Ethics Committee Approval / Material Supply Agreement
Institutional Ethics Approval was granted by the Southern Adelaide Clinical Human Research Ethics Committee (Application Numbers 449.14) and a Material Supply Agreement was executed by the Australian Red Cross Blood Service (ARCBS) (N15-02SA-03).
2.2 Preparation of PBS solution
A 0.0005 mol/L KH2PO4 + Na2HPO4, pH 7.4 + 10 mmol/L dextrose phosphate-buffered saline (PBS) solution was prepared as described in Sutera et al. (1985) with some modification.
2.3 Preparation of oxime solution
Pyridine-2-aldoxime-methochloride (Pralidoxime) was added to distilled water to make up a 600 mmol/L solution.
2.4 Collection of blood samples
Thirty one anonymous venous blood samples from South Australian (SA) donors were collected from a suburban donor during routine blood donation. Each donor was interviewed and provided informed consent. Blood samples were collected in heparin tubes (10mL) and immediately placed into an iced box for further measurements. The samples were collected on four different days with blood measurements performed within ten hours of blood collection.
2.5 Separation of plasma
1 mL from each of a blood sample was first pipetted to a separate tube for further ChE measurements. A blood sample with the rest blood was then centrifuged for 10 minutes at 3000 rpm to separate the plasma. Plasma and buffy coat were removed and plasma stored for further measurements. Pure isolated mixed erythrocytes were further processed as below.
2.6 Fractionation of erythrocytes into age cohorts
Pure isolated mixed erythrocytes were separated into two tubes, 1 mL in each tube to be further processed. To develop a simple field approach to separate erythrocytes into old and young cells, one tube of separated erythrocytes was gently mixed and centrifuged for 3 minutes at 10,000 rcf to fractionate the erythrocytes into age groups. Simple centrifugation allows mature erythrocytes with higher specific gravity to settle to the bottom and reticulocytes with lower specific gravity to settle at the top. After centrifugation 100 µL from the top, containing young erythrocytes, and 100 µL from the bottom, containing older erythrocytes, were slowly pipetted into separate tubes.
2.7 Washing the erythrocytes
To avoid interference with monocytes, leukocytes, reticulocytes and residual plasma, a second tube of isolated mixed erythrocytes was washed with the PBS solution at a 1:5 ratio followed by further centrifugation for 10 minutes at 3000 rpm. After centrifugation the buffer solution was aspirated and erythrocytes resuspended with equal volume of buffer solution for AChE measurement.
Fractionated young and old erythrocytes were washed with PBS solution at the 1:5 ratio and centrifuged further for 3 minutes at 2000 rcf. After centrifugation the buffer was aspirated and erythrocytes were resuspended with equal volume of buffer solution for AChE measurement.
2.8 Cholinesterase measurements
AChE measurements were conducted using the colorimetric Ellman method on the EQM Test-Mate ChE test system (EQM Research Inc 2003, Cincinnatti, US).
Twenty blood samples were measured for AChE activity in whole blood and mixed, young and old erythrocytes with and without oxime. Eight microliters of corresponding sample were added to a 10 µL capillary with 2 µL of either a PBS or oxime solution.
AChE in the remaining 11 samples was measured using both 8 µL and 10 µL of whole blood, and mixed, young and old erythrocytes.
Out of 31 blood samples, 27 samples were processed for measurements. Data was expressed as AChE (units/mL) and Q (units/g). AChE (units/mL) are directly comparable with population data that are generally published, whereas Q (units/g) is the ratio of AChE (units/mL) to haemoglobin (Hgb) (g/dL) and gives a standardised value taking into account blood volumes.
2.9 Statistical analyses
Data was analysed on GraphPadPrism v4 (GraphPad Software Inc.2003). Data was first analysed for normal distribution using the Shapiro-Wilk test for normality. All data was normal and homogenous or insignificantly different from normal distribution. Paired t-test and one-way ANOVA tests were used for comparisons (despite some data not being normally distributed, the tests are robust enough to handle slight variations from normality).
Correlation and linear regression tests were used to analyse the 8 µL and 10 µL blood measurements. Correction factors were derived to convert 8 µL volume measurements to 10 µL.
Paired t-test or one-way ANOVA for repeated measures test were performed.
Results were presented as means and standard deviations (±SD) for all groups.
3. Results
3.1 Correction of data to 10µL equivalent blood volumes.
Since measurements on the test-mate were performed using either 10 µL volumes of blood or 8 µL of blood + 2 µL of saline/oxime volumes, data must be corrected to yield equivalent values.
3.1.1 8µL and 10µL measurements
Table 3.1 shows the means ±SD of AChE (units/mL) and Q (units/g) data measured in 8 µL and 10 µL whole blood of 11 donors. All data was normally distributed.
Table 3.1: AChE (units/mL) and Q (units/g) Mean ±SD of 8 µL and 10 µL of whole blood measurements in 11 South Australians
|
Whole blood volume |
AChE (units/mL) |
Q (units/g) |
|||
|
Mean |
SD |
Mean |
SD |
||
|
8 µL |
2.834 |
±0.404 |
28.05 |
±2.62 |
|
|
10 µL |
3.730 |
±0.307 |
29.07 |
±2.33 |
|
Fig. 3.1a and 3.1b show the correlation of AChE measured in 8 µL and 10 µL in 11 samples represented as units/mL and Q (units/g). The slope and correlation factor (1/slope) in 8 µL and 10 µL measurements of AChE (units/mL) were found to be 0.932 and 1.073 respectively. The correlation was significant with r2 = 0.503. The slope and correlation factor (1/slope) in 8 µL and 10 µL measurements of AChE represented as Q (units/g) were found to be 0.972 and 1.029 correspondingly. The correlation was significant with r2 = 0.750.
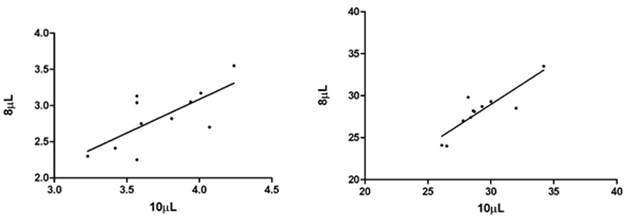
Fig. 3.1a. Correlation of 8 µL and 10 µL AChE Fig.3.1b. Correlation of 8 µL and 10 µL AChE
measurements in units/mL. measurements in Q (units/g).
3.1.2 Conversion of 8 µL to 10 µL measurements
The conversion of 8 µL measured whole blood and mixed, young and old erythrocytes has been made into 10 µL measurements using the correction factors for AChE (units/mL) 1.073 and Q (units/g) 1.029 and represented as AChE (units/mL) and Q (units/g). All following figures express data as 10µL equivalents.
3.2 Acetylcholinesterase in different age erythrocytes
AChE (units/mL) measurements in mixed, young and old erythrocytes of 17 donors are represented in Fig. 3.2a and 3.2b, and in Fig. 3.2c and 3.2d as Q (units/g). Fig 3.2a shows 8 µL erythrocytes measured, while Fig. 32b shows the 8µL measurements converted to 10µL.
Fig. 3.2c and 3.2d show the same AChE measurements represented in Q (units/g). The mean ±SD for AChE in 8 µL mixed, young and old erythrocytes were 3.492 units/mL (±0.747), 2.607 units/mL (±0.591) and 2.489 units/mL (±0.679). The mean ±SD for AChE in 10µL mixed, young and old erythrocytes were 3.746 units/mL (±0.802), 2.797 units/mL (±0.633) and 2.671 units/mL (±0.727). The mean ±SD for Q in 8 µL mixed, young and old erythrocytes were 27.62 (units/g) (±3.52), 25.56 (units/g) (±3.68) and 23.20 (units/g) (±3.55). The mean ±SD for Q in 10 µL mixed, young and old erythrocytes were 28.42 (units/g) (±3.63), 26.31 (units/g) (±3.79) and 23.87 (units/g) (±3.66).
The mean AChE of 8 µL and 10 µL measurements of mixed erythrocytes were significantly higher than those for both young and old erythrocytes (p<0.005), while both 8 µL and 10 µL mean AChE of young and old erythrocytes were not significantly different (p>005). The mean Q for 8µL and 10 µL in mixed erythrocytes were significantly greater than those in old erythrocytes (p<0.005). The other group means were not significantly different (p>0.005).
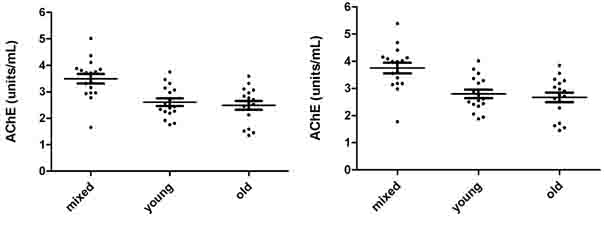
Fig. 3.2a. AChE in 8 µL mixed, young and old Fig. 3.2b. AChE in 10 µL mixed, young and old
erythrocytes in units/mL. erythrocytes in units/mL.
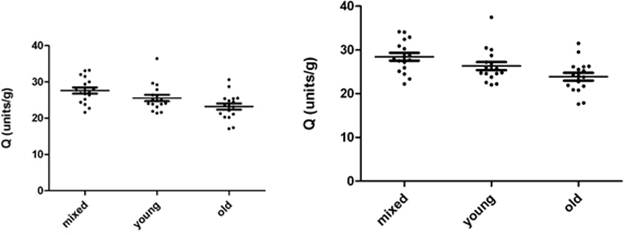
Fig. 3.2c. AChE in 8 µL mixed, young and old Fig. 3.2d. AChE in 10 µL mixed, young and old
erythrocytes, represented as Q (units/g). erythrocytes, represented as Q (units/g).
3.3 Acetylcholinesterase in oxime treated samples
3.3.1 Acetylcholinesterase in whole blood
Fig. 3.3a - 3.3d show AChE measurements in 17 donors whole blood treated and not treated with pralidoxime represented as units/mL and Q (units/g). Fig. 3.3a and 3.3c show the results of 8 µL samples for AChE (units/mL) and Q (units/g). Fig. 3.3b and 3.3d represent the AChE (units/mL) and Q (units/g) of 8 µL measurements converted to 10 µL. The mean ±SD for AChE in 8 µL of whole blood not treated with pralidoxime were 3.098 units/mL (±0.339) and treated with pralidoxime 2.514 units/mL (±0.204). These for 10 µL of whole blood not treated and treated with pralidoxime were 3.324 units/mL (±0.365) and 2.698 units/mL (±0.218) respectively. The mean ±SD for Q in 8 µL and 10 µL of whole blood treated and not treated with pralidoxime were 29.01 units/g (±3.17) and 23.04 units/g (±1.68), and 29.85 units/g (±3.26) and 23.70 units/g (±1.73) respectively.
Mean AChE and Q in oxime untreated (control) were significantly different for both 8 µL and 10 µL data (p<0.0001) from those of oxime treated groups.
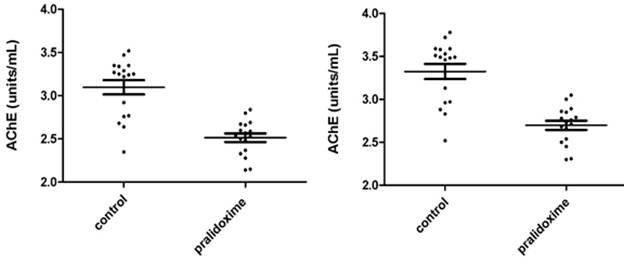
Fig. 3.3a. AChE in 8 µL whole blood treated and not Fig. 3.3b. AChE in 10 µL whole blood treated
treated with pralidoxime in units/mL. and not treated with pralidoxime in units/mL.
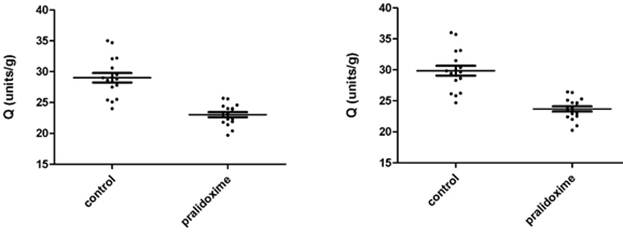
Fig. 3.3c. AChE in 8 µL of whole blood treated Fig. 3.3d. AChE in 10 µL of whole blood treated
and not treated with pralidoxime, represented and not treated with pralidoxime, represented
as Q (units/g). as Q (units/g).
3.3.2 Acetylcholinesterase in different age erythrocytes
AChE measurements in 17 donors’ mixed, young and old erythrocytes treated and not treated with pralidoxime are presented in Fig. 3.4a – 3.4d. Fig. 3.4a and 3.4c show AChE (units/mL) and Q (units/g) measurements of 8µL erythrocytes, and Fig.3.4b and 3.4d show converted 8 µL to 10 µL measurements of AChE (units/mL) and Q (units/g).
The mean ±SD for AChE in 8 µL of mixed, young and old erythrocytes that were not treated with pralidoxime were 3.492 units/mL (±0.747), 2.607 units/mL (±0.591) and 2.489 units/mL (±0.679) respectively, and for 8 µL of mixed, young and old erythrocytes treated with pralidoxime were 2.448 units/mL (±0.477), 2.061 units/mL (±0.330) and 2.026 units/mL (±0.449).
The mean ±SD for AChE in 10 µL of mixed, young and old erythrocytes not treated with pralidoxime were 3.746 units/mL (±0.802), 2.797 units/mL (±0.633) and 2.671 units/mL (±0.727); and treated with pralidoxime 2.627 units/mL (±0.512), 2.212 units/mL (±0.354) and 2.174 units/mL (±0.481).
The mean ±SD for Q in 8 µL of mixed, young and old erythrocytes not treated with pralidoxime were 27.62 units/g (±3.52), 25.56 units/g (±3.68) and 23.20 units/g (±3.55), and those for pralidoxime treated mixed, young and old erythrocytes were 20.08 units/g (±2.66), 20.46 units/g (±2.31) and 19.08 units/g (±2.23).
The mean ±SD data for Q in 10 µL mixed, young and old erythrocytes untreated with pralidoxime were 28.42 units/g (±3.63), 26.31 units/g (±3.79) and 23.87 units/g (±3.66), and for mixed, young and old erythrocytes treated with pralidoxime these were 20.66 units/g (±2.74), 21.06 units/g (±2.38) and 19.63 units/g (±2.29).
The effect of oxime on AChE between mixed, young and old erythrocytes in both 8 µL and 10 µL has found to be significantly different only in the mixed erythrocytes (p<0.05) with mean AChE in the control mixed erythrocytes being significantly higher than that in the pralidoxime treated mixed erythrocytes. The mean AChE in both oxime treated and untreated young and old erythrocytes were not significantly different from each other. When means for Q of controls and oxime treated groups in 8 µL and 10 µL measurements were compared in each erythrocyte age group (mixed, young and old), they were significantly different from each other (p<0.05).
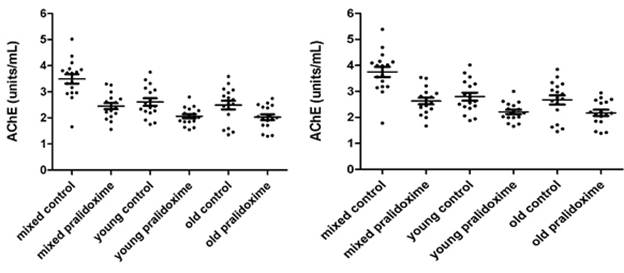
Fig. 3.4a. AChE in 8 µL mixed, young and old Fig. 3.4b. AChE in 10 µL mixed, young and old
erythrocytes treated and not treated with erythrocytes treated and not treated with
pralidoxime in units/mL. pralidoxime in units/mL.
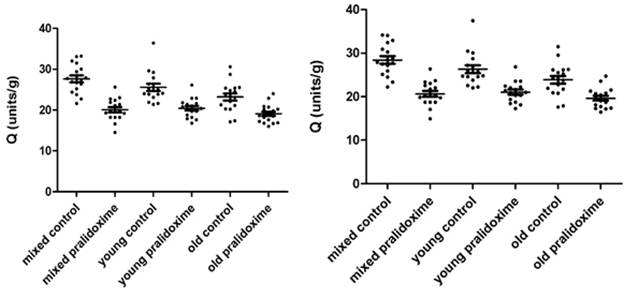
Fig. 3.4c. AChE in 8 µL mixed, young and old Fig. 3.4d. AChE in 10 µL mixed, young and old
erythrocytes treated and not treated with erythrocytes treated and not treated with
pralidoxime, represented as Q (units/g). pralidoxime, represented as Q (units/g).
4. Discussion
4.1 Introduction
This paper has focused on three major issues. First, regeneration of ChE enzymes in whole blood with oxime; second, assessing which erythrocyte age group has more AChE activity so provide a more sensitive index; and regeneration of different age erythrocyte cells by oxime.
4.1.1 8µL and 10µL measurements
Mean AChE (units/mL) and Q (units/g) of 8 µL measurements were found to be not significantly different to those of 10 µL blood measurements; both correlation factors were close to 1, especially for the Q (units/g) (Table 3.1, Fig. 3.1a-3.1b). Q (units/g) is the ratio of AChE (units/mL) to Hgb (g/dL), which represents AChE value taking into account different blood volumes. Despite this, 8 µL measurements were converted to 10 µL via correction factors to avoid any interfering and bias in the results and enable to compare the results with other papers using 10 µL blood volumes on the test-mate kit as well as the test-mate kit reference population data. Although, the results have been presented as both 8 µL and 10 µL measurements, further discussion will be of 10µL measurements only for clarity.
4.1.2 Consistency of sampling and analysis
Hgb concentrations in repeated samples can act as a surrogate indicator of reproducibility of this method. In this case Hgb concentrations of 8 µL measurements of whole blood and mixed, young and old erythrocytes were grouped into sample days and analysed. Mean values of each sample day within each group are not significantly different (p > 0.05). Hgb data in 78 measurements of 8 µL whole blood and mixed, young and old erythrocytes collected over four days were consistent. The consistency in Hgb data for each measurement suggests that pipetting and other techniques were performed correctly from measurement to measurement. Therefore, a technique based error can be eliminated as an influencing factor on obtained results.
4.2 Oxime regeneration of cholinesterase enzymes
4.2.1 Oxime
ChE enzymes are inhibited by OPs with brain ChEs being the target. Studies showed that ChE in parallel are affected in a similar way. Measurement of peripheral ChEs are used as biomarkers and proven to be good indicators of OP exposure and predictors for clinical effects (Mutch et al., 1992; Carlock et al., 1999; Hatijan et al., 2000; Mason, 2000; Dyer et al., 2001). However, studies on animals and humans have shown that both plasma ChE and AChE are not always inhibited by OP exposure (Maizlish et al. 1987; Bhatnagar et al.,1994; Plumlee et al.,1994; Parron et al. 1996). Farahat et al., 2003; Verma et al., 2009). The lack of one standard ChE measurement method might be a reason for such discrepancies found in the studies.
Ideally, pre-exposure (baseline AChE activity) and post-exposure values of AChE are required to accurately assess AChE inhibition. This would require several baseline measurements to establish statistically significant measurements. While statistical significance is obtained, biological significance of ChE inhibition is different. As described earlier, moderate exposure for a prolonged period may decrease AChE activity by 70% with no clinical symptoms produced, whereas single acute exposure causing 30% activity inhibition may cause symptoms with no previous exposure. Moreover, 20% inhibition in an individual with few baseline data available may not be statistically significant. It however becomes biologically significant if further even slight exposure occurs decreasing already depressed enzyme activity and causing symptoms.
While occupationally exposed workers might have their pre-exposure AChE measurements, the general population and others do not have such measurements in cases of single environmental exposure or accidental occupational exposure. In such cases, presumably exposed levels of AChE might be compared to the reference population distribution. However, there is a baseline variation of AChE activity with intraindividual variation in blood ChE activity of 6.3% for men and 6.7% for women (Sidell & Kaminiskis, 1975). Such a great variation in the AChE activity would only allow detecting statistically significant inhibition from 15% (Lotti, 1995). Thus, individuals with high levels of AChE within the normal distribution may have their enzyme inhibited but retain relatively high AChE and be incorrectly identified as ‘unexposed’. In contrast, those with normally low level of AChE activity might misleadingly be assessed as having their AChE inhibited.
The third approach to determine AChE depression is to measure the enzyme after its recovery. AChE recovery rate is associated with erythrocytes’ lifespan which is average 120 days (Prall et al., 2000). Some studies have shown that full recovery period is three months. For example, a study carried out on subjects over-exposed to OPs has found that plasma ChE was inhibited more than AChE immediately after exposure with an exponential pattern of recovery and a half-life for recovery of approximately 12 days (Fig.4.1a) (Mason, 2000), which is in accordance with other study findings (Dyer et al., 2001). In the same study AChE showed a linear activity recovery profile with 100% recovery in around 82 days (Fig.4.1b) (Mason, 2000). This study has also found that the theoretic baseline measurements for plasma ChE and AChE activity have to be done in 3 months after the exposure, suggesting that in this interval both enzymes would have returned to its pre-exposure levels (Mason, 2000). The disadvantage of this approach is that AChE inhibition cannot be confirmed until several months after the presumed exposure.
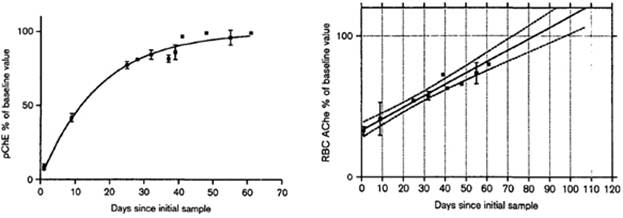
Fig. 4.1a. Recovery of plasma ChE activity in Fig. 4.1b. Recovery of AChE activity in
8 subjects. The mean ±SEM, measured at 8 subjects. The mean ±SEM, measured
each time-point, is shown for the subjects at each time-point, is shown for the subjects
(adapted from Mason, 2000). (adapted from Mason, 2000).
Oximes are strong nucleophiles able to reactivate OP-ChE conjugates. The efficacy of oxime reactivation depends on the structure and orientation of the nucleophile groups of the oximes and the structure of OP-ChE conjugate (Wong et al., 2000, Costa et al., 2011). Pralidoxime is the mono-pyridinium oxime widely used clinically against OP poisonings (Petroianu et al., 2006). In this study we attempted to regenerate AChE using pralidoxime to obtain an uninhibited baseline estimate against which oxime untreated AChE can be compared.
4.2.2 Plasma cholinesterase and acetylcholinesterase treated with oxime.
The results of pralidoxime treatment of AChE in whole blood have not shown reactivation of AChE but rather its inhibition. The AChE mean has decreased by about 23% after treatment with pralidoxime, and the Q mean also decreased by around 26% (Fig. 3.3b and 3.3d).
These results are not supported by other study findings showing reactivation of AChE by different oximes including pralidoxime (Worek et al., 1996, Jun et al., 2008, Costa et al., 2011). For example, the study by Costa et.al. (2011) tested four oximes on AChE inhibited by three different OPs. They have found that 1µL pralidoxime was capable to reactivate AChE by 10-14% after each OP inhibitor. Generally, the study demonstrated that with the increase of administered oxime concentrations (1µL-10µL-50µL-100µL) AChE reactivation was increased by up to 61%, although in vitro reactivation of ChE is not influenced by the oxime concentration. This is explained by the ageing process, which forms the AChE conjugate not able to be reactivated by the oxime (Costa et al., 2011). So, once oxime has reached and reactivated the AChE is no longer efficient if the ageing has occurred.
While, oxime had no regeneration effect on AChE, it increased plasma ChE measured in 12 of the current donors as part of a related project (Suratman, 2015) (Fig. 4.2) (samples for plasma ChE match those for AChE measurements). Each of the samples were measured twice either with 8 µL of plasma plus 2 µL of saline solution (controls) or 8 µL of plasma plus 2 µL of pralidoxime solution. 2 µL pralidoxime had reactivated plasma ChE by almost two fold. Although, the donors were not occupationally exposed to pesticides, the inhibited plasma ChE suggests the donors might have been domestically and/or accidentally exposed to OPs. The reactivation of plasma ChE allows assessing recent exposure to OPs.
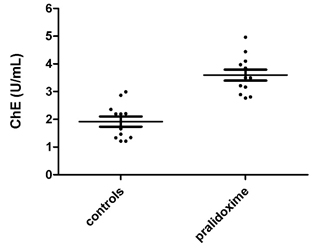
Fig. 4.2 ChE in 8 µL pure plasma treated and not with pralidoxime in units/mL.
In contrast, many studies have shown that oxime is a less effective reactivator of plasma ChE than AChE (Jun et al., 2008, Costa et al., 2011, Jun et al., 2011). In that study by Costa et al. (2011) none of the oximes was able to reactivate plasma ChE after the three tested OPs. Similarly, studies by Jun et al. (2008; 2011) have found a very low reactivation of plasma ChE from different oximes tested compared to that of AChE.
However, while these studies show less reactivation of plasma ChE, the results of the current study have shown the reactivation of plasma ChE by almost two times in each individual measurements of 12 random donors.
Ageing of the ChE enzymes was found to be one of the reasons for decreasing oxime reactivation (Wilson & Henderson, 1992). Depending on the type of OPs AChE cannot be reactivated or reactivation takes much longer if aging of an enzyme has occurred. Therefore, it could be suspected that the donors had been domestically or accidentally exposed to such types of OPs, which irreversibly inhibited the AChE. The presumed exposure was shown by the regenerated plasma ChE. Statistically, there is a slight possibility that all random 27 donors were exposed to such irreversible type of AChE inhibitors; nevertheless, it cannot be excluded as a possible factor. It can be suggested that future studies should take the exposure histories of donors into consideration to obtain a clearer picture of AChE behaviour. Furthermore, pralidoxime has different regeneration efficacy on AChE depending on the type of OPs (Jocanovic et al., 1995). Orientation of phosphoryl group in anionic site of inhibited AChE defines the effect of regeneration by oxime. Pyridinium oximes were reported to have a partial regeneration effect on AChE (Jocanovic et al., 1995). The other possible factor affecting ChE regeneration is formation of phosphorylated oximes, which further inhibit the enzyme (Worek et.al., 1999; Costa et.al., 2011; Jun et al., 2011). This may explain why AChE was inhibited by the pralidoxime in each measurement. Higher concentrations of both enzyme and oxime increase the probability of formation of phosphorylated oximes. Plasma ChE has a larger active site than AChE, which enables phosphorylated oximes to better accommodate and further inhibit regenerated enzyme (Worek et.al., 1999; Costa et.al., 2011). However, plasma paraoxonase is able to decompose some types of phosphorylated oximes (obidoxime and other pyridinium-4-aldoximes) reactivating AChE (Kiderlen et al., 2000), although more studies are required to confirm this, as human plasma appears to behave differently.
Similarly, a study on the five different oximes’ effect on AChE has found a little inhibition effect of pralidoxime on the AChE (Petroianu et al., 2006). While it is suggested that these results do not have any clinical relevance, in vivo studies are recommended to test the oxime effects as OP protective agents (Petroianu et al., 2006).
4.3. Acetylcholinesterase in erythrocytes
An attempt to discover the AChE pattern of activity and recovery in different erythrocyte age cohorts has been made. It was expected that young cell AChE would be more sensitive and susceptible to both inhibition and regeneration. AChE was first measured in mixed, young and old erythrocytes, regeneration of AChE by pralidoxime was then assessed in the same erythrocyte age groups.
4.3.1 Acetylcholinesterase in erythrocyte age cohorts
The results of this study have shown there was no significant difference between young and old AChE activity presented as both AChE (units/mL) and Q (units/g). Instead it was found that mixed erythrocytes had the highest AChE (units/mL) and Q (units/g). AChE represented in units/mL was higher in mixed cells compared to young and old cells; however, when presented in Q (units/g) it was only significantly higher compared to the old erythrocytes. Q represents AChE taking into account different blood volumes in the measurements, it is, therefore, a more accurate representative of the enzyme activity. Thus, it can be seen that there was significant difference in AChE activity in mixed cells (Higher activity) compared to old cells, but not between mixed and young or young and old erythrocytes.
A study by Prall et al. (2000) on the patients with chronic renal failure has similarly looked at the AChE activity in whole blood, purified mixed erythrocytes and different age erythrocytes groups with results expressed as Q (units/g). The results showed that AChE activity decreased from young to old erythrocytes by more than two times in controls, although the highest AChE activity has been shown in whole blood. In another study erythrocytes were separated in 8 age groups by centrifugation of the cells (Kadlubowski & Agutter, 1977). The in vivo ageing of erythrocytes identified a 21% decrease of AChE (Kadlubowski & Agutter, 1977). Other factors besides the reduction of catalytic activity with erythrocyte ageing may also explain the decrease in AChE activity. For instance, contamination of the young layer of the cells with reticulocytes, crypticity of the enzyme with erythrocyte ageing, and resealing of the older erythrocytes causing the blockage of the enzyme active site (Kadlubowski & Agutter, 1977).
Studies found that AChE decrease with cell aging were carried out using simple centrifugation to fractionate erythrocytes to different age groups. The disadvantage of this method is the poor separation of the erythrocytes, allowing only qualitative assessment of the AChE changes during erythrocyte senescence. A more advanced method on fractionating erythrocytes used by Galbraith and Watts (1981) enabled to discover a more complicated pattern describing AChE activity and cell age profile. The AChE activity is determined by both the amount of AChE, which decreases with cell age, and modifying effect on enzyme activity as a result of interaction with cell membrane aging. This tends to decrease from young to middle cells followed by further increase from middle to old cells (Galbraith & Watts, 1981; Lawson & Barr, 1987).
Although the current study used a simple erythrocyte separation method, the results demonstrated the complicated AChE pattern described by Galbraith & Watts (1981). Current research showed a slight increase in AChE activity in the younger cells comparing to the older cells with the highest AChE activity in mixed erythrocytes. It could be said that the mixed cells might have accumulated high AChE activity from both young cells (AChE amount) and old cells (AChE modifying effect).
It is more probable, however, that the fractionation method of erythrocytes used in the current study might be a reason for the mixed cells having the highest AChE activity. Simple centrifugation allows for the separation of erythrocyte cells based on the age-density association. However, it is speculated that young and old cells are still present in a top and bottom layer of the centrifuged mass (Leif & Vinograd, 1977). The middle layer of the centrifuged erythrocytes, which represent mixed cells, might simply accumulate a greater number of younger erythrocytes with higher AChE activity.
4.3.2 Acetylcholinesterase in oxime treated erythrocyte age cohorts
It was expected that erythrocyte group with a higher AChE activity would as well have AChE with a higher reactivation rate. The results of this section is consistent with the previous part results. Mixed erythrocytes in a control group have again shown the highest AChE represented in units/mL and Q (units/g). The oxime in erythrocytes had the same inhibition effect on AChE as in whole blood.
Pralidoxime was
shown to significantly inhibit AChE in mixed erythrocytes but made no
significant difference on AChE in young or old cells when presented in
units/mL. However, when presented as Q (units/g), oxime was shown to have a
significant effect in each erythrocyte group. This further supports the use of
Q as a more accurate and sensible index of AChE activity. Although the aim was
to find a correlation between activity and recovery patterns of the most
sensitive AChE in the younger erythrocyte cells, it was rather shown that mixed
erythrocytes had the highest AChE activity. This was further confirmed by the
highest inhibition of AChE in this erythrocyte group. Despite the oxime having
not regenerated AChE in either whole blood or erythrocytes, it could still show
that the highest AChE in mixed erythrocytes is more vulnerable to inhibition
The current study was able to show the consistency in the results in different
measurements.
Despite erythrocytes were isolated from the whole blood, it is possible that
platelets or white cells remained. Platelets and white cells have other
esterase (eg lymphocytes have NTE), which similarly as in whole blood, might
affect oxime effect leading to the AChE inhibition. At the stage of
erythrocytes isolation, platelets and white cells can be detected
microscopically to use further methods to remove them. Further studies would be
recommended using more advanced technique of erythrocytes isolation and
fractionation.
5. Conclusion
In conclusion, there are three approaches to detect AChE inhibition associated with some disadvantages. The first is assessment of post-exposure measurements with pre-exposure values, where pre-exposure levels of AChE are not usually available for the general population. The second is comparison of presumed inhibited AChE to the population reference distribution; however, high intraindividual variation in AChE might result in inaccurate assessment of an individual AChE. The third approach is assessment of AChE against its recovered level, where AChE cannot be measured during several month due to a long recovery period of the enzyme (three months).
Although this research was unable to regenerate AChE, the technique, regenerating AChE using oxime, remains potentially the most effective to accurately assess AChE inhibition. This would give opportunities for better measurement and interpretation of pesticide exposure allowing atropine application. This current technique would provide an ideal way to measure exposure in the field. Modifications to enable using a finger prick blood test to measure AChE with and without oxime in the field may be introduced to improve the exposure assessment.
Further research is required using a different type of oxime or oxime plus atropine treatment and/or erythrocyte fractionation method (eg gradient centrifugation) to better separate erythrocyte age cohorts and obtain a more accurate assessment of AChE activity and regeneration rates. The Ellman method is very convenient and rapid, although problems may still occur with regards to AChE measurement, for example erythrocyte heme interfering with thiocholine measurements (Coye et al., 1986), or other esterases interfering the AChE reading. Furthermore, pralidoxime was shown to have an effect on the Ellman’s reagent (Nadarajah, 1992). The field test-mate instrument has more application with plasma ChE measurement than AChE, as it is easier to separate and analyse plasma than erythrocytes, where other esterases or white blood cells might remain. However, the study on the repeatability and validity of the field test-mate kits has shown AChE is more repeatable than plasma ChE for surveillance measurements (London et al., 1995). It might still be recommended to use alternative method (ie. Hattachi) for AChE measurements in future work.
Additional work could be done using different washing solutions to preserve AChE activity in isolated cells. The PBS used in the current research was adapted from Sutera et al. (1985) with added salt. Although the possibility of washing solution damage on erythrocytes cannot be disregarded, several measurements were done to test the washing solution for its desired effect prior to its use on donor’s blood. Also, work could be orginised in such a way that the measurements of ChE enzymes are performed within a few hours after blood collection to avoid any time effects on enzyme activity. In the current work all samples were processed within 10 hours after collection. Thus, while timing could contribute to the true AChE activity, it is worth noting that the first sample measurements processed within an hour after the blood collection were identified to be similar to the last samples measurements in regards to AChE and Hgb data.
High temperature (30°C) during the blood centrifuging would allow free movement of the cells by decreasing blood viscosity and increasing cell deformability, resulting in improved separation process as recommended by Murphy (1973). The blood separation was performed at a constant room temperature (25°C). Whether this would have an effect on separation or not is unclear until further tested.
References
1. Allison, A & Burn, GP, 1955, “Enzyme activity as a function of age in the human erythrocyte”, British journal of haematology, vol. 1, pp. 291-303.
2. Bhatnagar,VK, Famxa, VKJ & Vora, AB, 1994, ‘Inhibition of cholinesterase in male mice fed dichlorvos (DDVP)’, Industrial Journal Toxicology, vol. 1, pp. 49–51.
3. Brown, LM, Blair, A, Gibson, R, Everett, GD, Cantor, KP, Schuman, LM, Burmeister, LF, Van Lier, SF & Dick, F, 1990, ‘Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota’, Cancer Res, vol. 50, pp. 6585-6591
4. Buckley, NA, Karalliedde, L, Dawson, A, Senanayake, N & Eddleston, M, 2004, ‘Where is the evidence for the management of pesticide poisoning - is clinical toxicology fiddling while the developing world burns?, Journal of Toxicology. Clinical Toxicology, vol. 42, pp. 113-116.
5. Calvert, GM, Plate, DK, Das, R, Rosales, R, Shafey, O, Thomsen, C, Male, D, Beckman, J, Arvizu, E & Lackovic, M, 2004, ‘Acute occupational pesticide-related illness in the US, 1998–1999: surveil- lance findings from the SENSOR pesticides program’, American Journal of Industrial Medicine, vol. 45, pp. 14–23.
6. Carlock, LL, Chen, WL, Gordon, EB, Killeen, JC, Manley, LS, Mullin, LS, Pendino, KJ, Percy, A, Sargent, DE, Seaman LR, Svanborg, NK, Stanton, RH, Tellone, CL & Van Goethem, DL, 1999, ‘Regulating and assessing the risks of cholinesterase-inhibiting pesticides: divergent approaches and interpretations’, Journal of Toxicology and Environmental Health, vol. 2, no. 2, pp. 105–160.
7. Chowdhary, S, Bhattacharyya, R, & Banerjee, D 2014, ‘Acute organophosphorus poisoning’, Clinica Chimica Acta vol 431, 66-76
8. Costa, LG, 1996, ‘Biomarker research in neurotoxicology: the role of mechanistic studies to bridge the gap between the laboratory and epidemiological investigations’, Environmental Health Perspectives, vol. 104, pp. 55-67.
9. Costa, MD, Freitas, ML, Soares FAA, Carraty, VS & Brandao, R, 2011, “Potential of two new oximes in reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by organophosphate compounds: An in vitro study”, Toxicology in Vitro, vol. 25, pp. 2120-2123
10. Coye, MJ, Lowe, JA & Maddy, KT, 1986, “Biological monitoring of agricultural workers exposed to pesticides: I. Cholinesterase activity determinations”, Journal of Occupational and Environmental Medicine, vol. 28, pp. 619-627
11. Dyer, SM, Cattani, M, Pisaniello DL, Williams, FM & Edwards, JW, 2001, ‘Peripheral cholinesterase inhibition by occupational chlorpyrifos exposure in Australia termiticide applicators’, Toxicology, vol. 169, pp. 177-185.
12. Eddleston, M 2000, ‘Patterns and problems of deliberate self-poisoning in the developing world’, Quarterly Journal of Medicine, vol. 93, pp. 715-731
13. Eddleston M, Karalliedde, L, Buckley, NA, Fernando, R, Hutchinson, G, Isbister, G, Konradsen, F, Murray, D, Piola, JC, Senanayake, N, Sheriff, R, Singh, S, Siwach, SB & Smit, L, 2002, ‘Pesticide poisoning in the developing world – a minimum pesticide list’, Lancet, vol. 360, pp. 1163-1167.
14. Edwards, JW, 2015, Personal communication, Flinders University, Adelaide, Australia
15. Elersek, T & Filipic, M, 2011, ‘Organphosphorous Pesticides – Mechanisms of their toxicity, in M Stoytcheva (ed) Pesticides– the impacts of pesticides exposure, Intech, pp. 243-260
16. EQM Research Inc, 2003, ‘Test-mate ChE Cholinesterase Test System (Model 400)’, Instruction manual, Ohio, USA
17. Farahat, TM, Abdelrasoul, GM, Amr, MM, Shebl, MM, Farhat, FM & Anger WK, 2003, ‘Neurobehavioural effects among workers occupationally exposed to organophosphorus pesticides’, Occup Environ Med, vol.60, pp. 272-286
18. Galbraith, D & Watts, D, 1981, “Human erythrocyte acetylcholinesterase in relation to cell age”, Biochem.J, vol. 195, pp.221-228
19. Griffin, P, Mason, H, Heywood, K, & Cocker, J, 1999, ‘Oral and dermal absorption of chlorpyrifos: human volunteer study’, Occupational and Environmental Medicine, vol. 56 pp
20. Grube, A, Donaldson, D, Kiely, T & Wu, L, 2011, Pesticides industry sales and usage 2006-2007 market estimate, US Environmental Protection Agency, Washington, viewed 13 May 2016 <http://www.epa.gov/opp00001/pestsales/07pestsales/market_estimates2007.pdf>.
21. Gunnell, D, Eddleston, M, Phillips, MR & Konradsen, F, 2007, ‘The global distribution of fatal pesticide self-poisoning: systematic review’, BMC Public Health, vol. 7, p. 357
22. Gupta, RC, 2006, Toxicology of Organophosphate & Carbamate Compound, Elsevier Academic Press.
23. Gupta, S, Stravitz, RT, Dent, P & Hylemon, PB, 2001, ‘Down-regulation of cholesterol-7alpha-hydroxylase (CYP7A1) gene expression by bile acids in primary rat hepatocytes is mediated by the c-Jun N-terminal kinase pathway’, J Biol Chem, vol. 276 (19), pp. 15816-15822
24. Hatijan, BA, Mutch, E, Williams, FM, Blain, PG & Edwards, JW, 2000, ‘Cytogenetic response without changes in peripheral cholinesterase enzymes following exposure to a sheep dip containing diazinon in vivo and in vitro’, Mutation Research, vol. 472, pp. 85-92
25. Hernandez, AF, Gomez MA, Perez V, Gacio-Lario, JV, Pena, G, Gil, F, Lopez, O, Rodrigo, L, Pino, G & Pla, A, 2006, ‘Influence of exposure to pesticides on serum components and enzyme activities of cytotoxicity among intensive agriculture farmers’, Environmental Research, vol. 102, pp. 70-76
26. Jaga, K & Dharmani, C, 2003, ‘Sources of exposure to and public health implications of organophosphate pesticides’, Rev Panam Salud Publica, vol. 14, no 3,pp. 171-185
27. Jokanovic, M, Maksimovic, M, Kilibarda, V, Jovanovic, D & Savic, D, 1995, “Oxime-induced reactivation of acetylcholinesterase inhibited by phosphoramidates”, Toxicology Letters, vol. 85, pp. 350-39
28. Jun, D, Musilova, L, Kuca, K, Kassa, J & Bajgar, J, 2008, “Potency of several oximes to reactivate human acetylcholinesterase and butyrylcholinesterase inhibited by paraoxon in vitro’, Chemico-biological interactions, vol. 175, pp. 421-424
29. Jun, D, Musilova, L, Musilek, K & Kuca, K, 2011, “In Vitro Ability of Currently Available Oximes to Reactivate Organophosphate Pesticide-Inhibited Human Acetylcholinesterase and Butyrylcholinesterase’, International journal of molecular sciences, vol. 12, pp. 2077-2087
30. Kadlubowski, M & Agutter, P, 1977, “Changes in the Activities of some Membrane‐associated Enzymes during in Vivo Ageing of the Normal Human Erythrocyte”, British journal of haematology, vol. 37,pp. 111-125
31. Kamel, F & Hoppin, JA 2004, ‘Asociation of pesticide exposure with neurologic dysfunction and disease’, Environ Health Perspect, vol. 112, no.9, pp. 950-958
32. Kiderlen, D, Worek, F, Klimmek, R & Eyer, P, 2000, “The phosphoryl oxime-destroying activity of human plasma”, Archives of toxicology, vol. 74, pp. 27-32
33. Lah, K, 2011, Pesticide Use Statistics, Toxipedia, April, viewed 1 May 2016
34. Lakew, K & Mekonnen, Y, 1998, ‘The health status of northern Omo State Farm workers exposed to chlorpyr- ifos and profenofos’, Ethiopian Medical Journal, vol. 36, no 3, pp. 175-184.
35. Lawson, AA & Barr, RD, 1987, ‘Acetylcholinesterase in red blood cells’, American Journal of Hematology, vol. 26, pp. 101-112.
36. Leif, RC &Vinograd, J 1977, ‘The distribution of buoyant density of cell membranes in viscometric flow: Behavior of intracellular and human erythrocytes in bovine serum albumin solutions’, Proc Natl Acad Sci USA, vol. 51, p. 520
37. Lein, PJ, Bonner, MR, Farahat FM, Olson, JR, Rohlman DS, Fenske, RA, Lattal, KM, Lasarev MR, Galvin, K, Farahat, TM & Anger, WK, 2012, ‘Experimental strategy for translational studies of organophosphorus pesticide neurotoxicity based on real-world occupational exposures to chlorpyrifos’, NeuroToxicology, vol. 33, pp. 660-668
38. Levin, ED, Ryde, IT, Seidler FJ, Slotkin TA & Tate, CA, 2006, ‘Organophosphate insecticides target the serotogenic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition’, Environmental Health Perspectives, vol. 114.10, pp. 1542-1555
39. London, L, Thompson, ML, Sacks, S, Fuller, B, Bachmann, OM & Myers, JE, 1995, “Repeatability and validity of a field kit for estimation of cholinesterase in whole blood”, Occupational and Environmental Medicine, vol. 52, pp. 57-64
40. Lotti, M, 1995, “Cholinesterase inhibitor: complexities in interpretation”, Clin. Chem., vol. 41, pp. 1814-1818
41. Maizlish, N, Schenker, M, Weisskopf, C, Seiber, J & Samuels, S, 1987, ‘A behavioral evaluation of pest control workers with short-term, low-level exposure to the organophosphate diazinon’, Am J Ind Med, vol. 12, no. 2, pp. 153–172.
42. Marrs, TC, 2007, “Toxicology of organophosphate nerve agents. Chemical warfare agents: toxicology and treatment” 2
43. Mason, HJ, 2000, ‘The recovery of plasma cholinesterase and erythrocyte acetylcholinesterase activity in workers after over-exposure to dichlorvos’, Occup. Med., vol. 50, no. 5, pp. 343-347
44. Mishra, GA, 2006, ‘The effect of tobacco consumption on blood cholinesterase levels among workers exposed to organophosphorus pesticides’, Toxicology and Industrial Health, vol. 22, pp. 399-403
45. Muldoon, SR & Hodgson, MJ, 1992, “Risk factors for nonoccupational organophosphate pesticide poisoning”, Journal of Occupational and Environmental Medicine, vol. 34, pp. 38-41.
46. Murphy, JR, 1973, “Influence of temperature and method of centrifugation on the separation of erythrocytes”, J. Lab. Clin. Med., vol. 82, pp. 334-341
47. Mutch, E, Blain PG & Williams FT, 1992, “Interindividual variations in enzymes controlling organophosphate toxicicity in man”, Human and Experimental Toxicology, vol. 11, pp. 109-116
48. Padilla, S, Wilson, VZ & Bushnell PJ, 1994, ‘Studies on the correlation between blood cholinesterase inhibition and ‘target tissue’ inhibition in pesticide-treated rats’, Toxicology, vol. 92, pp. 11-25
49. Parron, T, Hernández, AF & Villanueva, E 1996,’ Increased risk of suicide with exposure to pesticides in an intensive agricultural area. A 12-year retrospective study’, Forensic Sci Int, vol. 79, pp. 53–63.
50. Petroianu, G, Arafat, K, Kuca, K & Kassa, J, 2006, “Five oximes (K‐27, K‐33, K‐48, BI‐6 and methoxime) in comparison with pralidoxime: in vitro reactivation of red blood cell acetylcholinesterase inhibitied by paraoxon”, Journal of Applied Toxicology, vol. 26, pp. 64-71
51. Plumlee, KH, Richardson, ER, Gardner, IA & Galey, FD, 1994, ‘Effect of time and storage temperature on cholinesterase activity in blood from normal and organophosphorus insecticide-treated horses’, J Vet Diagn Invest, vol. 6, pp. 247–249.
52. Pope, CN, Chakraborti, TK, Chapman, ML, Farrar JD & Arthun, D, 1991, ‘Comparison of in vivo cholinesterase inhibition in neonatal and adult rats by three organophosphorothioate insecticides’, Toxicology, vol. 68, pp. 51-61
53. Prall, YG, Gambhir, KK, Cruz, IA, Blassingale, J & Ampy, FR, 2000, “Acetylcholinesterase activity in chronic renal failure”, Life sciences, vol. 66, pp. 835-845.
54. Pritchard, JA, 1949, “Erythrocyte age and cholinesterase activity”, American Journal of Physiology--Legacy Content, vol. 158, pp. 72-76.
55. Radcliffe, J. C. (2002). Pesticide use in Australia, Australian Academy of Technological Sciences and Engineering, viewed 23 April 2015
56. Ren, Z, Zhang, X, Wang, X, Qi, P, Zhang, B, Zeng, Y, Fu, R & Miao, M, 2014, ‘AChE inhibition: One dominant factor for swimming behaviour changes of Daphnia magna under DDVP exposure’, Chemosphere, vol. 120c, pp. 252-257
57. Roberts, DM & Brett, J, 2014, Clinical Management of Acute OP Pesticide Poisoning. Basic and Clinical Toxicology of Organophosphorus Compounds. Springer, pp. 141-175.
58. Sabine, JC, 1940, “Choline esterase of blood cells and plasma in blood dyscrasias, with special reference to pernicious anemia”, Journal of Clinical Investigation, vol. 19, pp. 833-842
59. Sidell, FR & Kaminiskis, A, 1975, ‘Temporal intrapersonal physiological variability of cholinesterase activity in human plasma and erythrocytes’, Clin Chem, vol. 21, no. 13, pp. 1961-1963
60. Slotkin, TA, Tate, CA, Ryde, IT, Levin ED & Seidler FJ, 2006, ‘Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition’, Environmental Health Perspectives, vol. 114, no. 10, pp. 1542-1546
61. Suratman, Ross, K, Babina, K & Edwards, J, PhD Thesis, 2015
62. Sutera, SP, Gardner, RA, Boylan, CW, Carroll, GL, Chang, KC, Marvel, JS, Kilo, C, Gonen, B & Williamson, JR, 1985, ‘Age-related changes in deformability of human erythrocytes’, American Society of Hematology, vol. 65, no. 2, pp. 275-282
63. Thiermann, H, Kehe, K, Steinritz, D, Mikler, J, Hill, I, Zilker, T, Eyer, P & Worek, F, 2007, ‘Red blood cell acetycholinesterase and plasma butyrycholinesterase status: important indicators for the treatment of patients poisoned by organophosphorus compounds’, Arh Hig Rada Toksikol, vol 58, pp. 359-366
64. U.S. Environmental Protection Agency, 2002, Chlorpyrifos; end-use products cancellation order”, doc, no: 01-2184, pp. 7753—7759, accessed 13 May 2016
65. Verma, SK, Kumar, V & Gill, KD, 2009, ‘An acetycholinesterase-independent mechanism for neurobehavioral impairments after chronic low level exposure to dichlorvos in rats’, Pharmacology, Biochemistry and Behavior, vol. 92, pp. 173-181
66. Waddell, BL, Zahm, SH, Baris, D, Weisenburger, DD, Holmes, F, Burmeister, LF, Cantor, KP & Blair, A, 2001, ‘Agricultural use of organophosphate pesticides and the risk of non-Hodgkin's lymphoma among male farmers (United States)’, Cancer Causes Control, vol. 12, no. 6, pp. 509-517
67. Wesseling, C. McConnell, R, Partanen, T, Hogstedt, C, 1997, ‘Agricultural pesticide use in developing countries: health effects and research needs’, Int. J. Health Serv., vol. 27, no. 2, pp. 273–308.
68. Wilson, BW & Henderson, JD, 1992, “Blood esterase determinations as markers of exposure” in GW Ware, Reviews of environmental contamination and toxicology, Springer-Verlag, vol. 128, pp. 55-69
69. Wilson, BW, Henderson, JD, Ramirez, A & O’Malley, MA, 2002, “Standardization of clinical cholinesterase measurements”, International journal of toxicology, vol. 21, pp. 385-388.
70. Wong, L, Radic, Z, bruggemann, RJ, Hosea, N, Berman, HA & Taylor, P, 2000, “Mechanism of oxime reactivation of acetylcholinesterase analyzed by chirality and mutagenesis”, Biochemistry, vol. 39, pp. 5750-5757
71. Worek, F, Kirchner, T, Backer, M & Szinicz, L, 1996, “Reactivation by various oximes of human erythrocyte acetylcholinesterase inhibited by different organophosphorus compounds. Archives of toxicology, vol. 70, pp. 497-503
Ïîñòóïèëà â ðåäàêöèþ 13.05.2016 ã.